B They both have the same number of electrons in their outermost orbital. The row starting with hydrogen is 1.
What Is The Position Of Helium In The Periodic Table Quora
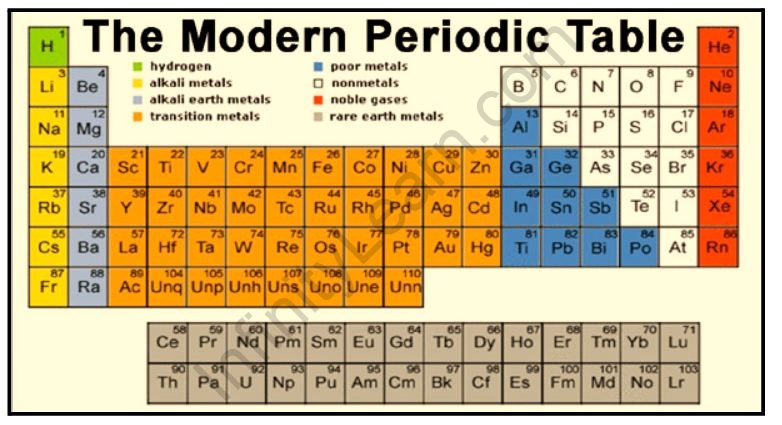
The main body of the table is a 18 7 grid.
. Since there are perhaps 1001 forms of the periodic table the answer depends on the perspective or properties of interest. Please note that the elements do not show their natural relation towards each other as in the Periodic system. These elements are placed below the periodic table of elements and are called the 4f series.
The row starting with lithium is 2. Why is He positioned above Ne in the periodic table. What substance is used in medicine to produce various images of organs and tissues.
So its electron configuration is. Referring to the atomic number of carbon attained from the periodic table how many neutrons does C14 have. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Is related to its position on the periodic table. On the periodic table hydrogen and helium are the only two elements in the first row or period which reflects that they only have electrons in their first shell. What substance is used in medicine to produce various images of organs and tissues.
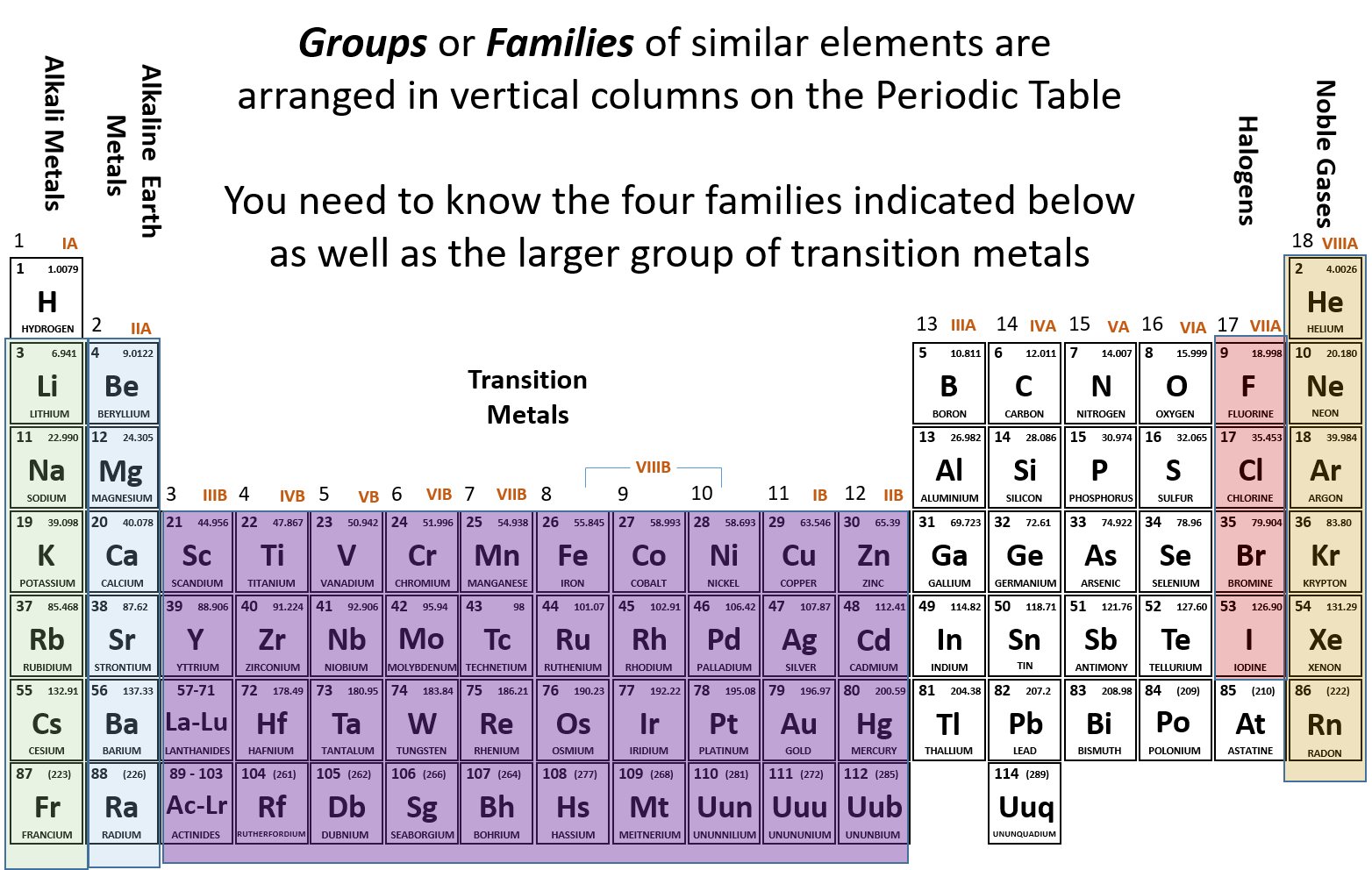
Helium is also found. A vertical column in the periodic table. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers electron configurations and chemical properties.
The smallest unit of an element that still retains the chemical and physical properties of that element is called an isotope. Group 8A or VIIIA of the periodic table are the noble gases or inert gases. Some of them are not found in nature but are man-made.
Its monatomic form H is the most abundant chemical substance in the Universe constituting. Phosphorus is in the third group in the p-block so it must have 3 electrons in the p shell Periods in the periodic table ESABO The following diagram illustrates some of the key trends in the periods. For details about provenance of the element property.
The second electron shell 2n contains another. A They both have the same atomic mass. They both have the same number of protons in their nuclei.
Learn more about D Block and F Block elements here in detail here. It is placed above Ne. Why is He positioned above Ne in the periodic table.
The periodic table only lists chemical elements and includes each isotope of each element within one cell. They both have the same number of electrons in their outermost orbital. Hydrogen and helium are the only two elements that have electrons exclusively in the orbital in their neutral non-charged state.
These are simple integer numbers. The number of protons in the nucleus increases as you go to the right or go down in the Periodic Table. For example H denotes hydrogen.
Period numbers are located on the left-hand side of the table. Elements are presented in increasing atomic number. Up to 10 cash back Helium and neon the two lightest noble gases have been traditionally positioned by IUPAC in the Group 18 of the Periodic Table of Elements together with argon and other unreactive or moderately reactive gaseous elements krypton xenon radon and oganesson.
The first chemical element is Cesium and the last one is Helium. Why is He positioned above Ne in the periodic table. C They both have a full outermost orbital.
Periodic table showing the relative sizes of the elements based on atomic radius data. The name comes from the Greek neos meaning new. Elements with the same number of valence electrons are kept together in groups such.
It is in group 18 noble gases of the periodic table. He has less atomic number than Ne. Actinides are all radioactive elements.
In the typical periodic table each element is listed by its element symbol and atomic number. Ne From Wikipedia the free encyclopedia Neon is a chemical element with symbol Ne and atomic number 10. He neon Ne argon Ar krypton Kr xenon Xe and radon Rn have full outer shells.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. They both have the same atomic mass. They are found in trace amounts in the atmosphere in fact 1 of the atmosphere is argon.
For chemistry students and teachers. Be has an atomic number of 4 and an atomic mass of 9. How to Identify It.
The periodic table is structured as an 18 X 7 grid positioned above a smaller double row of elements. The unity for ionization energy is eV. Effectively everyone is focussed on heliums status as a noble gas so it goes above neon in group 18 along with the rest of the noble gases.
The period indicates the highest energy level attained by electrons of an atom of the element in the ground state. In an atom the number of protons always equals the number of electrons. Trends on the periodic table.
This special periodic table shows the relative size of atoms of periodic table elements based on atomic radius data. Members of a group typically have similar properties and electron configurations in their outer shell. The atomic number of each element increases by one reading from left to right.
Why is He positioned above Ne in the periodic table. In this account we revive the old discussion on the possible placement of helium in. The periodic table is a chart of all the elements arranged in increasing atomic number.
Neon is a colorless odorless inert monatomic gas under standard conditions with about two-thirds the density of air. A horizontal row in the periodic table. Read more on Wikipedia.
They both have the same atomic number. The tabular chart on the right is arranged by Ionization energy. Referring to the atomic number of carbon attained from the periodic table how many neutrons does C14 have.
These actinides are also placed below the table in a row after lanthanides. Elements of the same period exhibit common characteristics and are placed. Of neutrons and protons.
Helium He neon Ne argon Ar krypton Kr xenon Xe and radon RnThe name comes from the fact that these elements are virtually unreactive towards other elements or compounds. Both are present in group-18. The number of circles in the electronic configuration of an element is represented in the periodic table as the period number that.
They both have a full outermost orbital. D They both have the same atomic number. Answer 1 of 8.
How many protons does it have. Note that the periodic table of elements page is provided in order to help navigate abundant chemical element data available in PubChem - each element also has a dedicated page with a lot more information available about each element including references. E They both have the same number of protons in their nuclei.
Long Form Of The Periodic Table Infinity Learn
What Is The Position Of Helium In The Periodic Table Quora
0 Comments